Introduction to Tramadol: A Comprehensive Overview
Tramadol, a centrally acting synthetic opioid analgesic, has garnered significant attention in the medical field due to its unique dual mechanism of action. This section provides an in-depth exploration of Tramadol’s pharmacology, mechanism of action, and classification, offering a clear understanding of its functionality and popularity in pain management.
Pharmacology of Tramadol
Tramadol is structurally related to codeine and morphine but operates differently from traditional opioids. It exists as a racemic mixture of two enantiomers: (+)-tramadol and (-)-tramadol, each contributing distinct analgesic effects. The drug is rapidly absorbed into the bloodstream after oral administration, with peak concentrations reached within 4.9 hours for sustained-release formulations. Its bioavailability is high, around 87-95%, making it effective for both acute and chronic pain management.
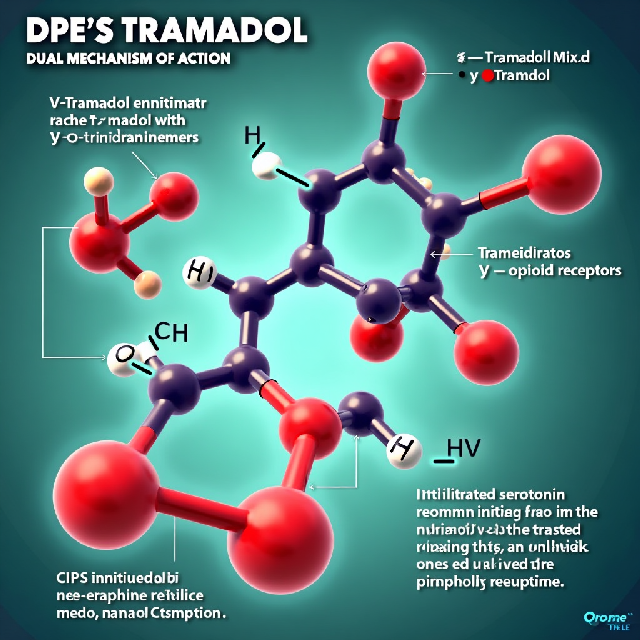
Dual Mechanism of Action
Tramadol’s effectiveness stems from its dual mechanism:
- μ-Opioid Receptor Agonism: The primary metabolite of tramadol, (+)-O-desmethyltramadol (M1), acts as an agonist at the μ-opioid receptors. This interaction is crucial for its pain-relieving properties.
- Monoamine Reuptake Inhibition: Tramadol inhibits the reuptake of serotonin and norepinephrine, neurotransmitters involved in pain regulation. (+)-Tramadol affects serotonin uptake, while (-)-tramadol impacts norepinephrine, enhancing the drug’s analgesic effects.
Classification as a Centrally Acting Synthetic Opioid
Tramadol is classified as a centrally acting synthetic opioid because it directly influences the central nervous system to alleviate pain. Unlike strong opioids, it has a lower affinity for opioid receptors, which reduces the risk of dependence and side effects like respiratory depression. This makes Tramadol a safer alternative for patients who cannot tolerate traditional opioids.
Therapeutic Uses
The unique pharmacological profile of Tramadol makes it versatile in treating various pain conditions:
- Acute Pain: Effective for postoperative pain relief.
- Chronic Pain: Useful in managing neuropathic pain and osteoarthritis.
- Special Populations: Ideal for elderly patients due to its favorable side effect profile compared to NSAIDs.
Safety Profile
Tramadol has a relatively low abuse potential and causes fewer side effects such as constipation and dependence compared to stronger opioids. However, it can interact with other medications, particularly those affecting serotonin levels, leading to conditions like serotonin syndrome. Monitoring for such interactions is essential for safe use.
In conclusion, Tramadol stands out due to its dual mechanism of action and favorable pharmacokinetics, making it a valuable option in pain management. Its unique balance of efficacy and safety has solidified its place as a widely prescribed analgesic.
Therapeutic Efficacy: A Closer Look
Tramadol is widely recognized as an effective medication for managing moderate to severe pain, making it a preferred choice in various clinical settings. Whether it’s alleviating post-surgical discomfort or addressing chronic pain conditions, Tramadol has proven its efficacy.
Post-Surgical Pain Relief
Tramadol is often prescribed after surgery because it effectively reduces pain without the need for stronger opioids, which can have more severe side effects. Studies show that patients who use Tramadol experience significant pain relief, allowing them to recover more comfortably and resume daily activities sooner. Its effectiveness in post-surgical scenarios highlights why it’s a go-to option for many healthcare providers.
Managing Chronic Pain
For those dealing with chronic conditions like arthritis or neuropathic pain, Tramadol offers long-term pain management with minimal adverse effects. Research indicates that it provides consistent relief across various chronic pain types, making it a versatile solution when other treatments fall short.
Preferred Over Other Medications
Tramadol stands out due to its dual-action mechanism, combining opioid and non-opioid effects for comprehensive pain relief. This unique profile reduces the need for higher-risk medications, aligning with current medical guidelines that prioritize safer alternatives. Its lower risk of dependency compared to traditional opioids further solidifies its position as a preferred treatment.
Effectiveness in Various Pain Conditions
- Neuropathic Pain: Tramadol is effective in managing shooting or burning nerve pain, often providing relief where other drugs fail.
- Musculoskeletal Pain: It’s commonly used for arthritis and lower back pain, offering consistent results with fewer side effects.
Key Takeaways
Tramadol’s versatility, safety profile, and proven effectiveness make it a top choice for moderate to severe pain management. Whether post-surgery or chronic conditions, Tramadol offers reliable relief, enhancing patient comfort and quality of life.
#Tramadol #PainManagement #Healthcare
Risks and Side Effects: Understanding Dependency, Addiction, and Physical Reactions
When exploring treatments or interventions, it’s crucial to understand the potential risks and side effects associated with them. This section delves into key areas such as dependency, addiction, respiratory depression, constipation, and skin reactions, providing insights into their implications and management.
1. Risk of Dependency
Dependency can arise from prolonged use of certain substances or interventions. Factors contributing to this risk include age, pre-existing conditions, and the potency of the substance. For instance, older adults may be more susceptible due to reduced metabolic rates, leading to higher concentrations of the substance in their system. Managing dependencies involves careful monitoring, gradual reduction, and alternative therapies to mitigate withdrawal symptoms.
2. Addiction Potential
Addiction is a severe side effect where individuals develop compulsive behaviors despite negative consequences. Substances like opioids have high addiction potential due to their impact on the brain’s reward system. Early signs of addiction include increased tolerance and withdrawal symptoms when attempting to cease use. Treatment often requires a combination of medication and behavioral therapy to address both physical and psychological aspects.
3. Respiratory Depression Risks
Respiratory depression is a life-threatening condition where breathing becomes shallow or stops. Opioids are notorious for this risk, especially in higher doses or when combined with other depressants like alcohol. Symptoms include slow breathing, blue-tinged skin, and loss of consciousness. Immediate medical attention is essential, often involving naloxone administration to reverse effects.
4. Constipation Side Effects
Chronic constipation can result from medications such as opioids, iron supplements, or calcium channel blockers. It leads to infrequent bowel movements, hard stools, and discomfort. Managing this includes dietary changes, hydration, and over-the-counter laxatives if necessary. Severe cases may require medical intervention to prevent complications like impaction.
5. Skin Reaction Dangers
Severe skin reactions, such as Stevens-Johnson Syndrome or Toxic Epidermal Necrolysis, can be life-threatening. Symptoms include painful rashes, blisters, and detachment of the skin. These reactions often require immediate hospitalization to manage pain, prevent infection, and promote healing.
Prevention and Management
- Monitor Usage: Track substance use carefully to avoid dependency and addiction.
- Consult Healthcare Providers: Discuss risks with professionals before starting any treatment.
- Report Symptoms Early: Seek help immediately if experiencing severe side effects like difficulty breathing or skin reactions.
By understanding these risks, individuals can make informed decisions and take proactive steps to protect their health. Share this information to raise awareness about the importance of cautious use and prompt medical intervention when necessary.
Regulatory Status and Classification: The Tramadol Landscape
Tramadol, a widely used synthetic opioid analgesic, has recently come under stricter regulatory scrutiny, impacting both medical practice and the world of sports. Here’s what you need to know:
1. Tramadol as a Schedule IV Controlled Substance
In 2014, the U.S. Drug Enforcement Administration (DEA) classified tramadol as a Schedule IV controlled substance under the Controlled Substances Act. This classification signifies that while tramadol has recognized medical uses, it carries a low potential for abuse and dependence compared to higher schedules like Schedule III. However, its placement in Schedule IV means prescriptions must be issued by a DEA-registered practitioner, either orally or in writing, and refills are generally prohibited without a new prescription.
2. Recent Addition of Tramadol to WADA’s Prohibited List
As of January 1, 2024, tramadol has been added to the World Anti-Doping Agency’s (WADA) list of prohibited substances for in-competition use. This decision was made following extensive research and discussions about its potential performance-enhancing effects and health risks for athletes. Studies suggest that tramadol can improve exercise performance by altering pain perception and mood, which could provide an unfair advantage. Additionally, its side effects, such as dizziness and nausea, pose safety concerns during competitions.
3. Implications of the Tramadol Ban for Athletes
- Therapeutic Use Exemptions (TUEs): Athletes requiring tramadol for medical reasons must now apply for a TUE, which involves submitting detailed documentation to justify its use.
- Health Risks: The ban highlights concerns about tramadol’s potential for addiction and physical dependence, even at therapeutic doses. Athletes are encouraged to explore alternative pain management strategies under medical supervision.
- Performance Impact: While some studies indicate that tramadol may enhance performance, others argue the evidence is inconclusive. Regardless, WADA’s decision underscores the need to maintain a level playing field and protect athlete health.
4. Regulatory Impact on Medical Practice
The scheduling of tramadol as a controlled substance has significant implications for healthcare providers:
- Prescribing Practices: Physicians must now adhere to stricter guidelines when prescribing tramadol, including limits on refills and requirements for patient monitoring.
- Patient Access: While the change aims to curb misuse, it could potentially limit access for patients with legitimate needs, particularly in regions with limited healthcare resources.
- Pain Management Alternatives: The medical community is encouraged to explore non-opioid alternatives for pain management to reduce reliance on tramadol and other controlled substances.
5. Balancing Regulation and Patient Care
The dual classification of tramadol as both a Schedule IV controlled substance and a WADA-prohibited substance reflects the delicate balance between ensuring patient access to effective pain relief and mitigating risks of abuse and unfair competitive advantages in sports. As regulations evolve, ongoing research and dialogue are crucial to address these challenges effectively.
Stay Informed, Share Responsibly
For athletes, coaches, and healthcare providers, understanding these changes is essential for compliance and ethical practice. Share this update with your network to help spread awareness about the evolving landscape of pain management and doping control.
#Tramadol #WADA #ScheduledSubstance #AthleteHealth #MedicalPractice
Controversies and Ethical Considerations: Balancing the Medical Benefits of Tramadol Against Its Potential for Misuse
The opioid crisis has brought the use of tramadol under intense scrutiny, highlighting a complex interplay between its medical benefits and societal risks. This section explores the debates surrounding opioid scheduling, ethical dilemmas in pain management, and the broader societal impacts of tramadol misuse.
Debates on Opioid Scheduling
Tramadol, classified as a Schedule IV controlled substance under the Controlled Substances Act (CSA) in the U.S., is considered to have a lower potential for abuse compared to Schedule II opioids like fentanyl or oxycodone. However, its scheduling has been a topic of debate. Some argue that stricter scheduling could reduce misuse, while others fear it may limit access for patients with legitimate needs.
Recent studies, such as those analyzing the rescheduling of hydrocodone from Schedule III to Schedule II, suggest that stricter scheduling can decrease overall prescriptions but may not significantly impact acute pain management. This raises questions about whether such measures effectively curb misuse without hindering medical use.
Ethical Dilemmas in Pain Management
Pain management is fraught with ethical challenges, particularly the undertreatment of pain and the risk of opioid addiction. Healthcare providers often face difficult decisions balancing the relief of suffering against the potential for harm.
-
Patient Autonomy vs. Addiction Risk: Patients have the right to effective pain relief, but prescribers must weigh this against the risk of addiction. This dilemma is compounded by varying definitions of “appropriate” use and the subjective nature of pain.
-
Undertreatment of Pain: Studies show that certain populations, including the elderly and those with chronic conditions, often receive inadequate pain management due to fears of opioid misuse. This undertreatment can lead to poorer health outcomes.
Societal Impacts of Tramadol Misuse
While tramadol is less frequently misused than other opioids, its potential for abuse still poses societal challenges:
-
Contribution to the Opioid Epidemic: The opioid crisis, responsible for tens of thousands of deaths annually, has raised concerns about any opioid’s role in misuse. Tramadol’s lower risk does not eliminate it from this discussion.
-
Vulnerable Populations: Regions with limited healthcare access often report higher rates of tramadol misuse, suggesting that socioeconomic factors play a significant role.
Balancing Medical Benefits Against Misuse
Striking the right balance requires multifaceted strategies:
- Prescription Monitoring Programs (PMPs): These can help identify patterns of misuse and overprescribing.
- Education: Both prescribers and patients need better education on opioid risks and benefits.
- Alternative Pain Management: Non-opioid therapies, such as NSAIDs or interventional procedures, should be considered to reduce reliance on opioids.
Conclusion
The use of tramadol reflects broader challenges in pain management and substance misuse. While it offers significant medical benefits, its potential for abuse necessitates careful consideration and comprehensive strategies to mitigate risks without denying patients necessary relief.
By addressing these issues through policy, education, and innovation, we can navigate the complexities of opioid use responsibly.
Future Research Directions: Exploring Optimal Dosages, Safer Formulations, and Market Growth in the Tramadol Drug Market
The Tramadol market is evolving rapidly, driven by advancements in medical research and growing demand for effective pain management solutions. Future studies are expected to focus on three key areas: optimizing dosages for better efficacy and safety, developing alternative formulations to reduce dependency risks, and analyzing market growth trends to guide industry strategies.
-
Optimizing Tramadol Dosages
Research into dosage optimization is critical to enhancing the therapeutic effects of Tramadol while minimizing side effects. Recent studies have explored personalized dosing regimens based on factors such as patient weight, renal function, and pain severity. For instance, a study published in the British Journal of Anaesthesia found that a dose of 2.5 mg/kg for pediatric patients provided effective postoperative pain relief with minimal adverse effects. Similarly, pharmacokinetic models, such as physiologically based pharmacokinetic (PBPK) models, are being used to predict how Tramadol is metabolized in different patient populations, enabling more precise dosing recommendations. These advancements could lead to tailored treatment plans that improve outcomes and reduce risks. -
Alternative Formulations to Reduce Dependency Risks
To address concerns about Tramadol’s potential for misuse and dependency, researchers are developing alternative formulations. Extended-release tablets and sustained-release compositions are being tested to maintain therapeutic effects with fewer doses, thereby reducing the risk of overuse. For example, a bilayer tablet formulation combining immediate-release (IR) and sustained-release (SR) layers has shown promise in providing rapid pain relief while maintaining longer-term efficacy. Additionally, novel delivery systems, such as biodegradable polymeric ribbons, are being explored to optimize drug release patterns and improve patient safety. -
Market Growth Trends Analysis
The global Tramadol market is projected to grow significantly over the next decade, driven by increasing demand for pain management solutions in an aging population and rising cases of chronic pain conditions. According to a report by Fortune Business Insights, the market size is expected to reach $3.50 billion by 2032, growing at a CAGR of 5.8%. This growth is further supported by advancements in drug formulations and the expansion of healthcare infrastructure in emerging markets. Companies are investing heavily in research and development to capture a larger share of this lucrative market.
Conclusion
The future of Tramadol research lies in striking a balance between efficacy, safety, and accessibility. By optimizing dosages, developing safer formulations, and understanding market dynamics, stakeholders can ensure that Tramadol remains a valuable tool in pain management while addressing its potential risks. As the market continues to expand, innovation will play a key role in shaping the next generation of Tramadol-based therapies.
The Importance of Responsible Tramadol Use: A Balanced Approach
Tramadol, a widely used opioid analgesic, is effective for managing moderate to severe pain but requires careful handling. Its misuse can lead to significant health issues, including dependence and side effects like seizures. Here’s a structured approach to ensure safe and effective use:
1. Responsible Use
- Patient Monitoring: Healthcare providers should closely monitor patients on Tramadol, especially those with histories of substance abuse.
- Avoidance of Alcohol: Patients must avoid alcohol while on Tramadol to prevent enhanced sedation and other complications.
- Gradual Dosage Reduction: To avoid withdrawal symptoms, gradual tapering is essential when stopping treatment.
2. Balanced Prescribing Practices
- Guideline Adherence: Doctors should follow clinical guidelines to assess patients and periodically re-evaluate therapy needs.
- Proper Assessment: Ensure the right patients receive Tramadol, avoiding those at high risk of complications.
3. Minimizing Risks
- Side Effect Awareness: Inform patients about potential side effects and steps to mitigate them.
- Safe Dosage Limits: Adhere to recommended doses to prevent overdose risks.
- Drug Interactions: Be cautious with medications that lower seizure thresholds or interact negatively with Tramadol.
4. Maximizing Benefits
- Combination Therapy: Using Tramadol with paracetamol can enhance pain relief while minimizing opioid use.
- Appropriate Indications: Reserve Tramadol for suitable pain types to optimize effectiveness and safety.
5. Informed Research
- Stay Updated: Continuous research is crucial to understand Tramadol’s long-term effects and refine usage guidelines.
- Evidence-Based Practices: Encourage studies on optimal dosing, side effect management, and alternative therapies.
By adopting these strategies, we can enhance the benefits of Tramadol while safeguarding against risks. Prioritizing responsible use and informed practices ensures patient safety and effective pain management.
I’m interested in knowing how the article discusses long-term risks from Tramadol due to its dual mechanism, which would help understand its dangers beyond just addiction.
@Fern wants more info on how Tramadol might cause long-term health problems beyond addiction. The article mentions some side effects but doesn’t cover the lasting effects on serotonin and norepinephrine systems or metabolism risks, which matter for patient safety.
I’m curious how the article discusses Tramadol’s long-term effects on serotonin and norepinephrine systems due to its dual-action mechanism. Does it compare Tramadol’s safety to other opioids because of how it works differently? Also, does the study suggest dosing strategies to reduce risks with long-term use?
I’m curious how the article discusses Tramadol’s long-term effects on serotonin and norepinephrine systems due to its dual-action mechanism. Does it compare Tramadol’s safety to other opioids because of how it works differently? Also, does the study suggest dosing strategies to reduce risks with long-term use?